Over USD10 Billion Estimated To Implement 5-Year WHO Draft Programme Of Work
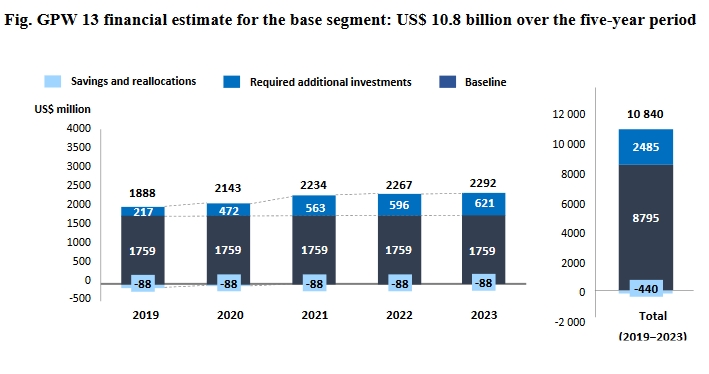
The World Health Organization released a financial estimate for its draft thirteenth general programme of work 2019-2023 on 15 January, after member states asked last autumn how that draft programme was to be financed.








