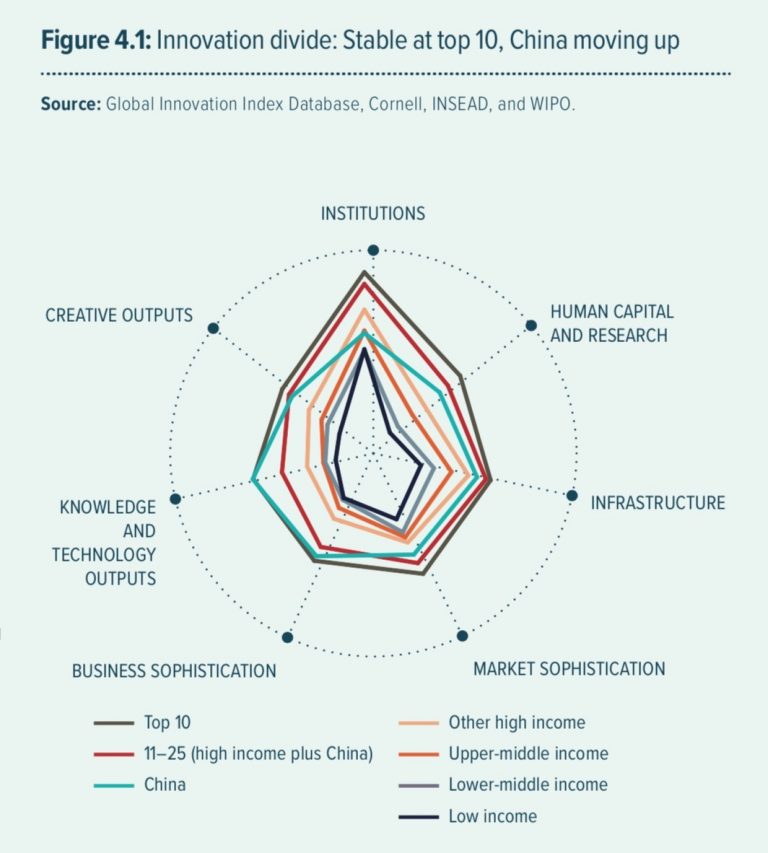
Members of the United Nations concluded negotiations on the draft of the Political Declaration on the Fight Against Tuberculosis on 20 July. After weeks of heated negotiations over the inclusion of references to TRIPS flexibilities in the operative paragraphs, with the Group of 77 pushing for inclusion and the United States against it, the final text of the political declaration reflects the deadlock of these positions. Due to the inability of member states to reach agreement, the final text does not include substantive reference to TRIPS flexibilities.
If no countries object, this final draft of the Political Declaration on TB will be adopted by the General Assembly at the High-Level Meeting on Tuberculosis, which will take place on 26 September at the United Nations in New York, and will serve as the authoritative agreement from which action plans will be drawn. According to sources, countries have until tonight in New York to decide whether to object, and G77 nations are considering their options.