WHO, Netherlands To Hold Fair Drug Pricing Forum In May
The World Health Organization and The Netherlands government will co-host a meeting in the spring that brings experts together to look at high drug prices and the purchasing of medicines.

Original news and analysis on international IP policy
The World Health Organization and The Netherlands government will co-host a meeting in the spring that brings experts together to look at high drug prices and the purchasing of medicines.
The US Army has extended the comment period on the proposed licence to pharmaceutical company Sanofi on technology necessary to create a vaccine for the Zika virus. This is the second extension, and will permit public comments through 10 March 2017.
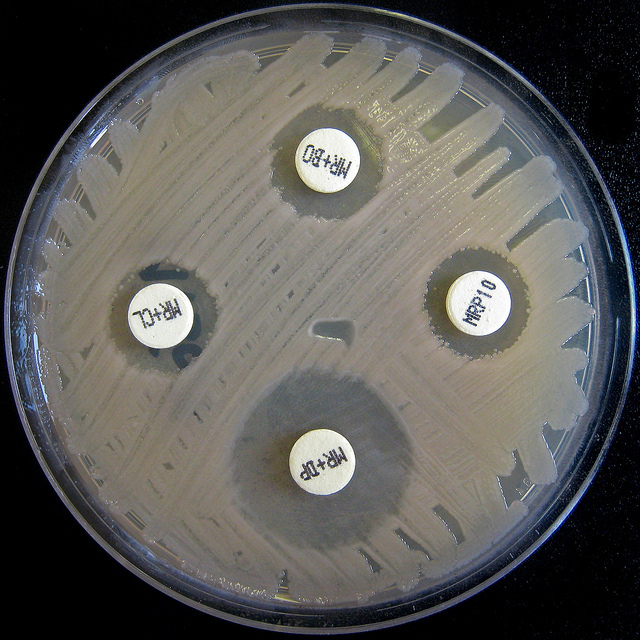
The rising threat of “super bugs,” bacteria resistant to existing antibiotics, was in discussion at the World Health Organization this week. Concerns were voiced about the slow pace of national action plan implementation to improve the careful use of antibiotics. Meanwhile some developing countries and civil society called for priority to be given to accessibility and affordability to new antibiotics.
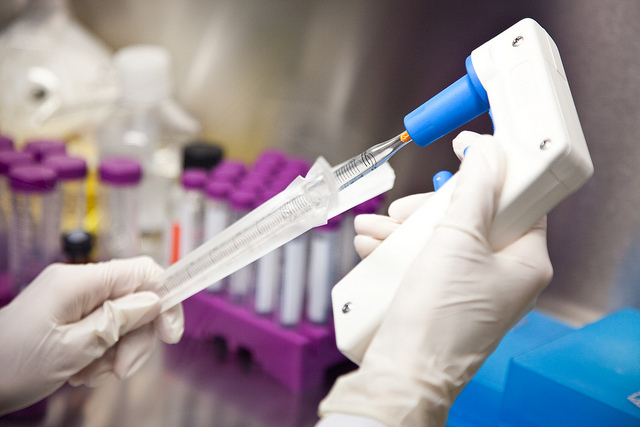
The Ebola outbreak spurred actions from the World Health Organization in terms of how to deal with emergencies and also getting medicines and vaccines to patients in emergency situations. The secretariat presented an implementation plan for the International Health Regulations, and a report on its recent blueprint on research and development for potentially epidemic diseases at its Executive Board meeting this week. The United States sought to limit the scope of WHO's work on R&D in this context.
The Medicines Patent Pool announced today that it has signed a license agreement with Johns Hopkins University for a candidate tuberculosis treatment. Although seen as a major step forward by public health groups, they said the agreement does not include guarantees that the treatment that could be brought to the market would be affordable for all.

At the opening of the World Health Organization Executive Board meeting today, a call by India for an agenda item on the report of the United Nations Secretary-General’s High-Level Panel on Access to Medicines was denied. Meanwhile, WHO’s director general underlined the success of the organisation over the last year, including new financing arrangements with industry groups to finance the WHO Prequalification Programme. But all eyes are riveted to the election process for the new WHO director general, as three out of the six candidates are expected to be short-listed this week.
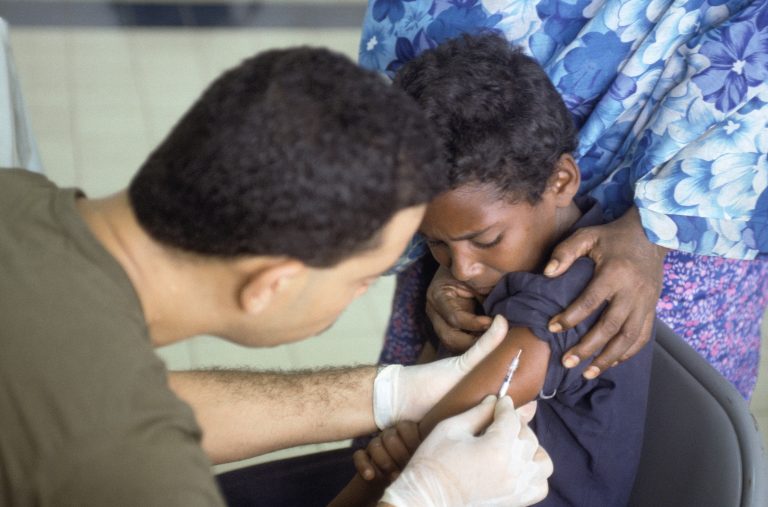
A range of civil society organisations have issued a public statement opposing the United States Army’s proposed grant of an exclusive licence on technology necessary to produce a Zika vaccine to French pharmaceutical company Sanofi. The letter cites concerns that the exclusive licence might violate US law and could lead to high priced medicines as consumers buy back taxpayer-funded research.
The World Health Organization recently published its analysis about the public health implications of the Nagoya Protocol on genetic resources access and benefit-sharing, and in particular how it affects the sharing of pathogens, like influenza viruses. The findings are set to be discussed at this month’s WHO Executive Board meeting. Also to be discussed is an experts group review of the WHO pandemic influenza framework, and in particular its conclusion that the framework should be amended to match scientific progress.

The rapid development of information and communication technologies, and the need for greater interconnectivity driven by the Internet of Things has created a variety of standard-essential patent (SEP) owners and implementers with different business models, and for more diverse licensing practices, the European Commission Joint Research Centre says in a new study. This has made it harder to agree on an interpretation of FRAND licensing principles, something European policymakers must clarify in order to meet key digital single market and other goals, it says.

A recently published book by a high-impact public health advocate provides new analysis on the use of flexibilities in international trade law relating to intellectual property rights aimed at advancing discussions on solutions to high drug prices worldwide.