New MPP-Gilead License For HIV Treatment
The Medicines Patent Pool today announced a new licence with Gilead Sciences for a medicine which in combination with others would offer a once-daily, single-tablet HIV treatment.

Original news and analysis on international IP policy
The Medicines Patent Pool today announced a new licence with Gilead Sciences for a medicine which in combination with others would offer a once-daily, single-tablet HIV treatment.

Today WIPO Director General Francis Gurry and Thomas Cueni, Director General of the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA), signed an agreement establishing the Patent Information Initiative for Medicines, or “Pat-INFORMED,” during the General Assemblies of the Member States of WIPO taking place this week. IFPMA worked with Intellectual Property Watch to prepare a Q&A with Mr Gurry and Mr Cueni about this new initiative.

Innovation is vital for the development of medicines, but innovation without proper access to them is pointless, Roberto Azevêdo, Director-General of World Trade Organization has said. Several other agency heads spoke at the same event, where World Health Organization Director General Tedros Adhanom Ghebreyesus stressed the importance of universal health coverage.

The annual World Intellectual Property Organization General Assembly opened today for 10 days during which delegates have to agree on budget, and on several decisions which might lead to more normative activities on the part of the organisation, although impatience is not equally shared depending on subjects. Separately, the United States submitted a new proposal on the budget, and the European Union tabled a proposal on a new mandate for the WIPO genetic resources and traditional knowledge committee.

Does the US Constitution prohibit the USPTO from striking down issued patents? That question will be decided by the US Supreme Court later this term. Should the Court rule against the USPTO, it would dramatically alter the US patent system in favor of patentees, give a big boost to patent trolls, and damage innovation in the US. The ruling also would make the US an outlier among major industrialized countries – turning it into the only such nation where patents could not be challenged in administrative proceedings.

The government of Japan, in partnership with the African Region Intellectual Property Organisation (ARIPO), has begun implementation of a project meant to train 1,000 people across Africa in intellectual property systems. The World Intellectual Property Organization also plays a role.

Initially, the lawsuit was widely viewed as a waste of time. The suit asserted a strained legal argument that already had been rejected twice by federal appellate panels, in 1985 and 1992. Yet this lawsuit, Oil States Energy Services v. Greene’s Energy Group, has now reached the US Supreme Court. So later this term, the high court will decide whether the US Constitution prevents the US Patent and Trademark Office from ever striking down issued patents.
“There are shameful access disparities around the world” to life-saving medicines, Harvard University Global Access in Action project Co-Director Quentin Palfrey said at a 26 September Center for Strategic and International Studies event in Washington, DC. And while some of the challenges to fuller access involve pricing, getting medicines to poorer countries or populations means overcoming the obstacles of insufficient research and development (R&D) incentives, access barriers and polarised politics, he said.
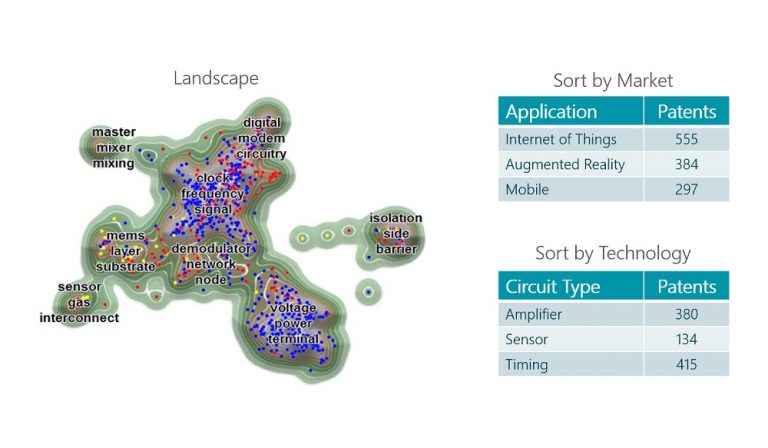
By Martin Bijman, Director, Intellectual Property Products , TechInsights Successfully pursuing the monetization of IP assets requires an accurate assessment of their value and position within the marketplace. Essentially, monetizing a patent portfolio includes four key steps: Developing an accurate…

The topic of access to medicines has gained momentum in recent years as high prices of new medicines affect developing countries and developed countries. The role of competition legislation in preventing market abuses and monopoly situations has been pointed to as a possible lever to facilitate access to generic medicines and balance the potential negative effects of intellectual property protection.