European Patent Office Report Compares Compulsory Licensing Practices By Country
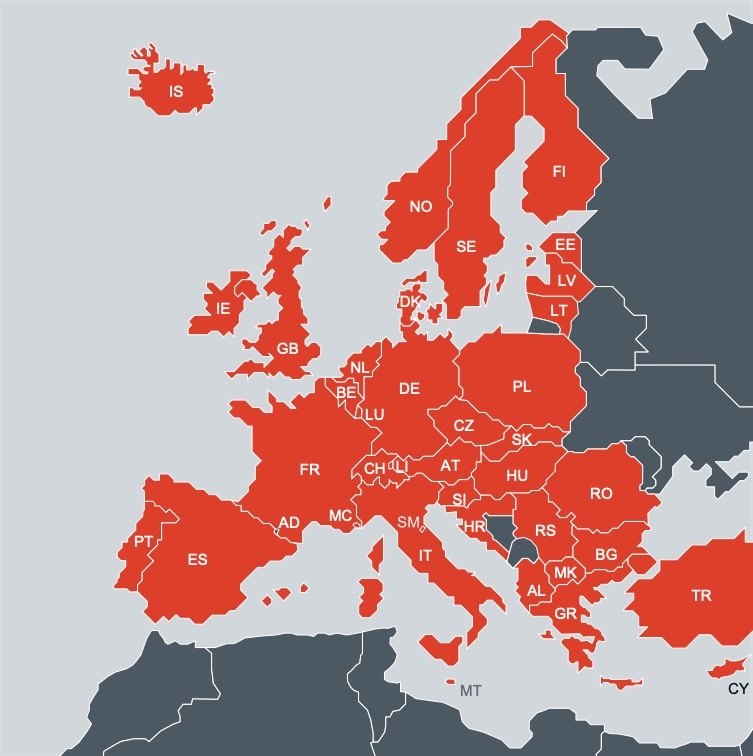
The European Patent Office has published a report detailing differences in the laws and procedures of European countries for the granting of compulsory licences.

Original news and analysis on international IP policy

The European Patent Office has published a report detailing differences in the laws and procedures of European countries for the granting of compulsory licences.

NEW YORK -- Artificial intelligence, robots, and the law, are all changing a rapid pace. A panel of experts at a recent event at Fordham Law School discussed latest developments and signs of the limits of the law when applied to AI areas like facial recognition, automated weapons systems, and financial technology.
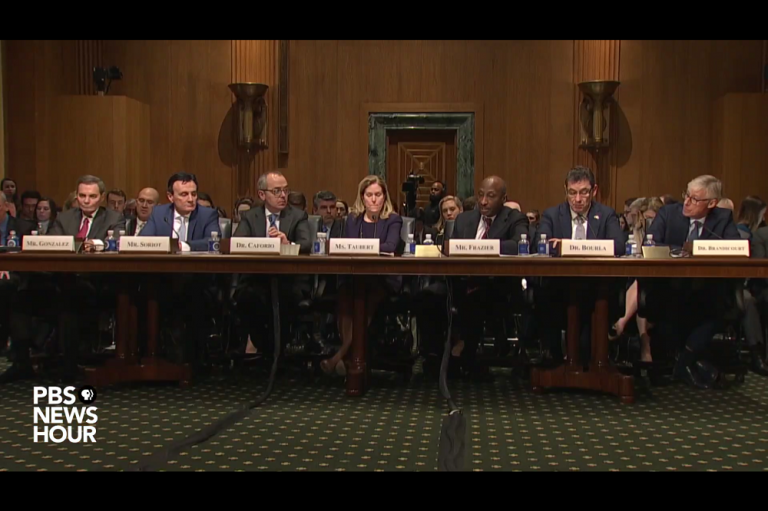
Executives of seven large pharmaceutical companies faced questioning yesterday from the United States Senate Finance Committee over high drug prices in the US, especially compared with other developed countries. One issue that came under the microscope was patents.

The Regional Comprehensive Economic Partnership (RCEP) trade agreement under negotiation among Asia and Pacific nations must not include measures that would undercut countries' ability to protect diverse local farming systems and sustainable plant genetic resources for food and agriculture, a range of Asian nongovernmental organisations argue. Groups in India, Malaysia and the Philippines this week specifically called for the RCEP not to include the high-level protections under the International Union for the Protection of New Varieties of Plants (UPOV).

Since 2003, Drugs for Neglected Diseases Initiative (DNDi) has worked to meet the public health needs of neglected populations by filling gaps in drug development left by the for-profit pharmaceutical industry. A new research study by the French Development Agency analysed DNDi’s unique product development partnership (PDP) model, and found that it “illustrate[s] what can be presented as a ‘commons’ within the area of public health.”

Dilip Shah was passionately committed to the twin causes of promoting fair access to medicines around the world, and to the success of the local Indian pharmaceutical industry. A bright light is out, writes Prof. Frederick Abbott.

Dilip Shah, founder of the Indian Pharmaceutical Alliance (IPA) the organisation that represented the interests of Indian drug makers passed away in Mumbai on Friday. He was 77. Known in the industry as "DG", Mr Shah started the IPA when the Indian pharma companies were trying to find their feet in the global pharma landscape that was dominated by the multinational pharma companies predominantly based out of Europe and USA. Shah who himself spent most of his career with the MNC drug companies ...

Ellen 't Hoen writes: On 1 February 2019, article 53(3), second sentence of the Dutch Patent Act 1995 came into force introducing a patent exemption for the preparation of medicines in a pharmacy.

Brainbase, a California tech startup with a team in Estonia, has received US$1M in seed funding to build an "end-to-end product ecosystem for intellectual property licensing" that could change the way businesses manage and licence their brands worldwide. The investment is an indication of dynamic evolution in the IP sector as it takes advantage of latest technologies and the talents of innovators.

The European Council of member states has adopted an all-encompassing plan to make Europe a global leader in artificial intelligence and integrate AI into all aspects of regional life. The plan, which comes as Europe has been identified as lagging in AI research and investment behind the United States and China, includes strong calls to ramp up investment while carefully considering AI's impact on labour and ethics.