
US President Signs New Trade Secrets Law
US President Barack Obama yesterday signed into law a measure aimed at strengthening trade secret protection including by allowing federal courts to hear cases involving trade secret theft.

Original news and analysis on international IP policy

US President Barack Obama yesterday signed into law a measure aimed at strengthening trade secret protection including by allowing federal courts to hear cases involving trade secret theft.

Public health advocates last week told World Health Organization delegates they must act quickly to save the lives of poor populations suffering from less common diseases for which there is no research and development funding. Nongovernmental organisations showed up to a WHO meeting on the issue to urge on delegates, even holding a public demonstration in front of the UN, but there was concern afterward at the little progress made.

New technologies are of limited value if they are not accessible. Thus the crucial challenge lies not only in promoting innovation, but in translating innovation into social impact. This was the theme of the fourth Conference on Technologies for Development (“Tech4Dev”).

The World Intellectual Property Organization, a United Nations agency, today gave the UN in New York an update of ongoing negotiations for the protection of indigenous knowledge and genetic resources.
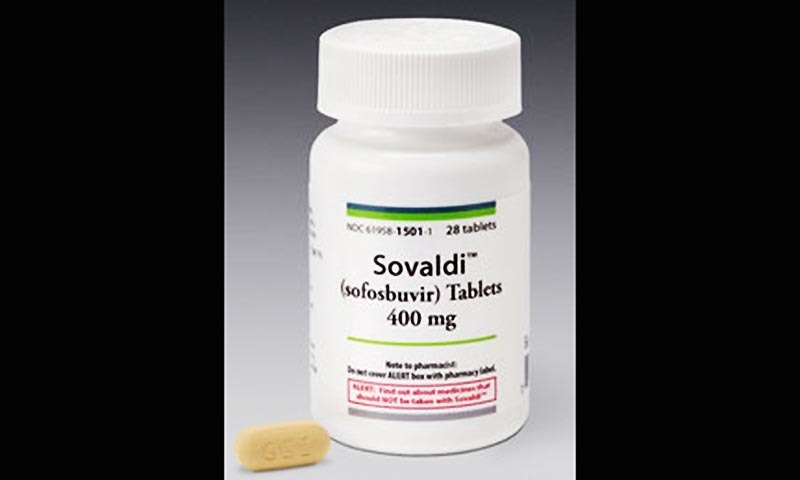
A copy of the new Indian patent office order shows the details of the decision to reverse an earlier direction and grant a patent in India on the high-value hepatitis C drug. [Updated with response from Gilead]

A number of World Health Organization member states attended a meeting last week aimed finding ways to sustainably finance research and development for medical products, especially those for poor populations lacking means to pay high prices. According to the outcome document and a WHO official, they heard many viewpoints from experts and made progress but much was left for the World Health Assembly later this month.

NAIROBI, KENYA -- A recent report by the United Nations Economic Commission for Africa (UNECA) is calling for faster establishment of a Pan-African Intellectual Property Organisation (PAIPO) to bring about what it sees as badly needed IP policy coherence on the continent.

The bipartisan heads of several United States congressional subcommittees have sent a letter urging the Obama administration to obtain the full and uncensored United Nations report on an investigation into possible misconduct by the head of the World Intellectual Property Organization. Meanwhile, procedural wrangling may be taking place within WIPO on who has the right to suppress or see the report.

This week's high-profile alleged leak of recent texts of the Transatlantic Trade and Investment Partnership (TTIP) negotiation between Europe and the United States sent a shockwave in policy circles. Below, Intellectual Property Watch highlights some of the IP-related elements in the text.
Médecins Sans Frontières (MSF, Doctor Without Borders) today announced a report detailing what it calls failings in the current system for developing new drugs in ways that all patients can afford and access, and providing proposed policy options for addressing the problems.

[story updated] Biosimilar drugs hold out big opportunities for India’s drug companies. But the future is fraught with challenges. One key challenge is regulations.
What does this mean for countries like India, an emerging market for biosimilars? How will the evolving global regulatory environment on biosimilars impact patients?
Last month, both these questions came to the fore as the battle over biosimilars moved centre stage in this country in the wake of an interim order by the Delhi High Court, and then another decision by a Division Bench of the same court which took a different view.

The World Health Organization interacts with a large number of actors aside from governments, such as industry, philanthropic organisations, academia, and civil society. With an eye to preventing undue influence on the work of the organisation, member states have been trying to finalise a draft framework on WHO interaction with those actors. This week, what was seen as a last effort at reaching a consensual text did not quite meet the goal and some additional informal discussions are expected to take place before the annual World Health Assembly in late May.